Chemical properties of gallium - Health effects of gallium - Environmental effects of gallium
|
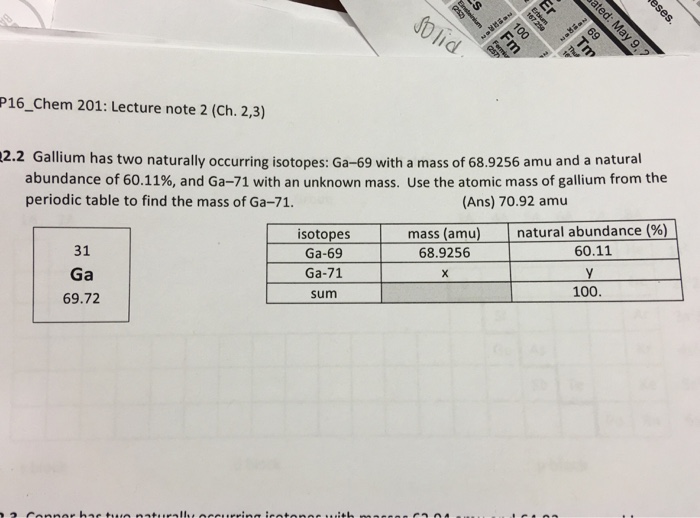
The gram atomic mass for gallium is 69.72. 'Mass number' is a property of isotopes, not of elements, and gallium has two radioactively stable isotopes, with mass numbers of 69 and 71. Gallium took the place of the placeholder element eka-aluminum. Gallium was first identified using spectroscopy by its distinct pair of violet spectral lines. Gallium's melting point (302.93 K) is low enough to melt the metal in the palm of your hand. Gallium is the element with the highest range of temperatures for its liquid phase.
GalliumSolid gallium is a blue-gray metal with orthorhombic crystalline structure; very pure gallium has a stunning silvery color. Gallium is solid at normal room temperatures, but as well as mercury, cesium, and rubidium it becomes liquid when heated slightly. Solid gallium is soft enough to be cut with a knife. It is stable in air and water; but it reacts with and dissolves in acids and alkalis. Applications Liquid gallium wets porcelain and glass surfaces; it forms a bright, highly reflective surface when coated on glass. It can be used to create brilliant mirrors. Gallium easily alloys with most metals, so it is used to form low-melting alloys. The plutonium pits of nuclear weapons employ an alloy with gallium to stabilize the allotropes of plutonium. Gallium does not exist in pure form in nature, and gallium compounds are not a primary source of extraction. Gallium is more abundant than lead but much less accessible bacause it has not been selectively concentrated into minerals by any geological process, so it tends to be widely dispersed. Several ores, such as the aluminum ore bauxite, contain small amount of gallium, and coal may have a relatively high gallium content. Health effects of galliumGallium is an element found in the body, but it occurs in a very small amount. For example, in a person with a mass of seventy kilograms, there are 0.7 milligrams of gallium in the body. If this amount of gallium was condensed into a cube, the cube would only be 0.49 millimeters long on one side. It has no proven benefit towards the function of the body, and it most likely is only present due to small traces in the natural environment, in water, and in residue on vegetables and fruits. Several vitamins and commercially distributed waters have been known to contain trace amounts of gallium with less than one part per million. Pure gallium is not a harmful substance for humans to touch. It has been handled many times only for the simple pleasure of watching it melt by the heat emitted from a human hand. However, it is known to leave a stain on hands. Even the gallium radioactive compound, gallium [67Ga] citrate, can be injected into the body and used for gallium scanning without harmful effects. Although it is not harmful in small amounts, gallium should not be purposefully consumed in large doses. Some gallium compounds can actually be very dangerous, however. For example, acute exposure to gallium(III) chloride can cause throat irritation, difficulty breathing, chest pain, and its fumes can cause even very serious conditions such as pulmonary edema and partial paralysis.
|
More from 'Elements'
Lenntech (European Head Office)
Distributieweg 3
2645 EG Delfgauw
The Netherlands
Phone: +31 152 610 900
fax: +31 152 616 289
e-mail: info@lenntech.com

Lenntech USA LLC (Americas)
5975 Sunset Drive
South Miami, FL 33143
USA
Phone: +1 877 453 8095
e-mail: info@lenntech.com
Atomic Number And Mass Chart
Lenntech DMCC (Middle East)
Level 5 - OFFICE #8-One JLT Tower
Jumeirah Lake Towers
Dubai - U.A.E.
Phone: +971 4 429 5853
e-mail: info@lenntech.com
Copyright © 1998-2021 Lenntech B.V. All rights reserved
| Isotope | Atomic mass (Da) | Isotopic abundance (amount fraction) |
|---|---|---|
| 69Ga | 68.925 573(8) | 0.601 08(50) |
| 71Ga | 70.924 702(6) | 0.398 92(50) |
In 1961, the Commission recommended Ar(Ga) = 69.72, based on the chemical ratio determinations as well as the isotope-abundance determinations. Recalculating the chemical ratios based on current values of the other atomic weights involved yields Ar(Ga) = 69.735, while the mass-spectrometric value with current atomicmasses gives Ar(Ga) = 69.72. Furthermore, highly precise coulometric assayof Ga and As yielded Ar(Ga) = 69.737. Meanwhile, new mass-spectrometricmeasurements confirmed the earlier mass-spectrometric values, yielding Ar(Ga) =69.724(2). Facing with this dataset, the Commission recommended an atomic weight of Ar(Ga) = 69.723(4) in 1983 favouring the mass-spectrometric data.
Significant variations occur in the n(69Ga)/n(71Ga) ratio of commercially high-purity Ga from different lots ofmaterial and different manufacturers, some exhibiting ratios 0.19 % higher and 0.12 % lower than thelaboratory reference material. Based on this information, in 1987 the Commission recommended Ar = 69.723(1), which has remainedunchanged since that time.
Purification of Ga by successive recrystallizations is accompaniedby small variations in isotopic composition, which measurably affect the triple-point temperature of gallium.
Atomic Mass Of Gallium 71
© IUPAC 2003
CIAAW
Gallium
Ar(Ga) = 69.723(1) since 1987
The name derives from the Latin gallia for France. It was discovered in zinc blende by the French chemist Paul-Emile Lecoq de Boisbaudran in1875. It was first isolated in 1878 by Lecoq de Boisbaudran and the French chemist Émile-Clément Jungflesch.
Isotopic reference materials of gallium.
